FASI Investigator Explores the Body’s Neuroimmune Connection

Scientific progress requires both developing new tools and finding the right research problem in which to apply them. Stephen Liberles, Ph.D., a Food Allergy Science Initiative (FASI) investigator who is also a professor in the cell biology department at Harvard Medical School and a Howard Hughes Medical Institute investigator, began his career as a chemist, a choice he partially attributes to the influence of his father, a theoretical chemist who studied molecular orbital theory.
Today, the discoveries he is making through his research on neuroimmune response are shedding new light on the relationship between the senses of sight, hearing, taste, touch and smell and food allergies.
However, he didn’t begin his career focusing on food allergies. As a graduate student, he focused on creating chemical tools – small molecules called dimerizers that can bring proteins together to elicit cellular signaling events – but strived to find the best application for them. The turning point in his career came when he became fascinated by sensory neuroscience and body-brain communication, and more generally decided to focus on biological questions of interest, using whatever tools might be at his disposal or even creating new ones as needed.
As a postdoctoral fellow with Nobel Laureate Linda Buck, Dr. Liberles turned his attention to olfaction and taste to understand how diverse chemicals are recognized by sensory neurons. One of his first big discoveries was identifying a second family of olfactory receptors called trace amine-associated receptors (TAARs). He found that TAARs detect several odors called amines, which are profoundly aversive. They included cadaverine, a death-associated odor; phenylethylamine, a carnivore odor that makes mice run away; and trimethylamine, an odor that causes the disease “fish malodor syndrome.”
“TAARs provided a really terrific model system to understand how the brain encodes aversive stimuli,” he said.
Beyond Smell and Taste
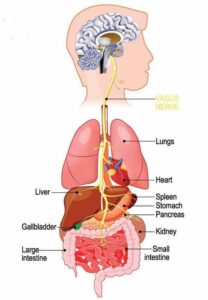
After discovering the TAARs – as well as a new family of receptors in the vomeronasal organ, a secondary olfactory organ involved in pheromone responses – Dr. Liberles turned his attention to finding sensory receptors involved in body-brain communication. He became fascinated by the vagus nerve, a group of neurons that innervates many vital organs in the body like the lungs, heart, stomach and intestine.
Vagus nerve research was dominant in the early 1900s, leading to several major breakthroughs in neuroscience and physiology, like the discovery of the first neurotransmitter acetylcholine.
“Core principles in neuroscience were derived from studies of the vagus nerve in the early 1900s, but in the recent molecular and genetic era, the vagus nerve kind of fell off the radar,” Liberles said. “As a result, our understanding of how this nerve worked significantly lagged behind that of our external senses of smell, taste, touch, vision and hearing. We were excited to revisit it and start using molecular and genetic tools to really understand how the vagus nerve relays information from the body. There are probably additional body-brain communication pathways that have remained hidden and are not understood so far by the field.”
The Neuroimmune Connection
By focusing on interoception – how the brain receives input from the body across physiological systems – Liberles’ lab hopes to gain insight into how the relationship between the gut and the brain can cause allergic reactions.
“We’ve been interested in understanding gut-brain signaling as it relates to, for example, food intake and nutrient sensation,” he said. “We’ve been interested in airway-to-brain sensation – how you detect when the lungs stretch or when irritants make you cough. We’ve also been interested in signals from the cardiovascular system – for example, changes in blood pressure, blood volume or respiratory gas levels.”
Liberles is now interested to see how many pathways for neuroimmune crosstalk there are, and how allergens activate the immune system differently from infection. “The idea that there are different types of sensory neurons that may be engaged across immune challenges is really exciting,” he said.
Broad Applications
The charting of cell types across the gut-brain axis and the study of neuroimmune interaction are two topics that Liberles said he believes will help FASI achieve its mission of curing food allergies, but there are other areas where his team’s findings are applicable across medicine, he said.
“I’m a basic scientist at heart. Interesting results may generalize in helpful ways, or alternatively, will provide unique wrinkles to contrast how different systems work,” he said. “I’m excited by general principles of neuro-immune interactions and specific wrinkles of allergen responses that will emerge from our work supported by FASI.”
FASI is the organization leading the way to the discovery of innovative therapeutic developments that could address the root cause of food allergies, and we’re proud to invest in scientists like Stephen Liberles. The only way we can advance his work, and that of our other investigators and discover potential treatments, is through your continued support.